Da Vinci Stapling-robotic Surgery-da Vinci Instruments & Accessories-medical-equipment-south-africa
Da Vinci Stapling
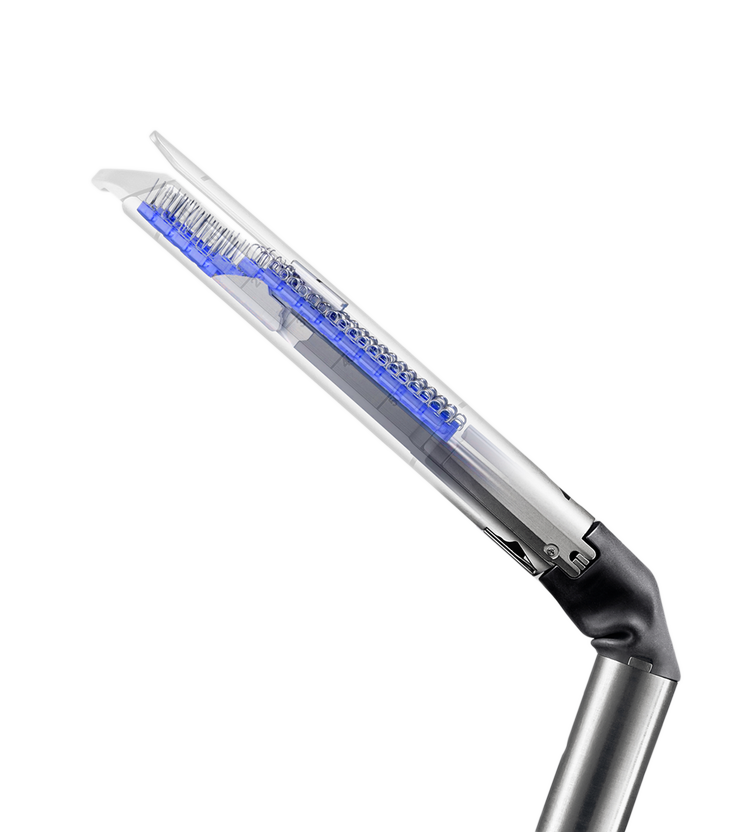
Staple with complete control, full articulation, and intelligent feedback
Advancing stapling
Position around vital structures and access deep, confined spaces with fully wristed da Vinci staplers—a first among leading laparoscopic staplers.(1) You can achieve transection and resection with control and autonomy working from the da Vinci surgeon console. Da Vinci OS4 software-enabled capabilities provides constant monitoring and real-time feedback to inspire confidence with every firing.
SureForm 45 and 60
At SureForm’s core is SmartFire technology which monitors tissue compression before and during firing. Combined with a 120° cone of articulation, you can reduce uncertainty and increase stapling performance like never before.(2)
EndoWrist Staplers 30 and 45
Take advantage of an integrated robotic stapling solution with complete control at the surgeon console, EndoWrist technology for fully wristed articulation, and SmartClamp technology for intraoperative feedback. Choose from a range of stapler lengths, tip profiles, and reload colors.
Evidence for robotic-assisted stapling
See an industry-first publication that provides technological evaluations of the EndoWrist Stapler matched to clinical outcomes of interest. The study reports on the performance of da Vinci stapling during robotic-assisted right colectomy.
References:
(1). Compared to EndoGIA Ultra with Tri-Staple technology, SIGNIA with Tri-Staple 2.0 technology, and Powered Echelon Plus with GST as of March 2019. Results from internal testing on file.
(2). Results from internal testing on file.
Disclosures and Important Safety Information
Important Safety Information
Serious complications may occur in any surgery, including da Vinci surgery, up to and including death. Examples of serious or life-threatening complications, which may require prolonged and/or unexpected hospitalization and/or reoperation, include but are not limited to, one or more of the following: injury to tissues/organs, bleeding, infection and internal scarring that can cause long-lasting dysfunction/pain.
Risks specific to minimally invasive surgery, including da Vinci surgery, include but are not limited to, one or more of the following: temporary pain/nerve injury associated with positioning; a longer operative time, the need to convert to an open approach, or the need for additional or larger incision sites. Converting the procedure could result in a longer operative time, a longer time under anesthesia, and could lead to increased complications. Contraindications applicable to the use of conventional endoscopic instruments also apply to the use of all da Vinci instruments.
For Important Safety Information, indications for use, risks, full cautions and warnings please also refer to www.intuitive.com/safety.
Individuals’ outcomes may depend on a number of factors, including but not limited to patient characteristics, disease characteristics and/or surgeon experience.
Instrument & Accessory Care
It is the responsibility of the owner of the da Vinci Surgical System to properly train and supervise its personnel to ensure that the instruments and accessories are properly cleaned, disinfected and sterilized as required by the User’s Manual. The da Vinci products should not be used in a clinical setting unless the institution has verified that these products are properly processed in accordance with the da Vinci System User’s Manual.
Xi System Stapler Family
The EndoWrist Stapler 30 and 45 Instruments and Reloads are intended to be used with the da Vinci Xi surgical system (IS4000) for resection, transection, and/or creation of anastomoses in General, Thoracic, Gynecologic and Urologic surgery.
The EndoWrist Staplers 30 and 45 are indicated for adult use, and the EndoWristStapler 30 is indicated for pediatric use. The devices can be used with staple-line or tissue-buttressing materials. The EndoWrist Stapler 30 and 45 Instruments and Reloads should not be used on tissue such as the liver or spleen, where tissue compressibility is such that clamping of the instrument would be destructive. Do not use the EndoWrist Stapler 30 and 45 Instruments or Reloads on the aorta.
The EndoWrist Stapler 30 and 45 for the da Vinci Xi System (IS4000) are not compatible for use with the da Vinci, da Vinci S, or da Vinci Si Surgical surgical systems.
Xi System SureForm Stapler 45 and 60
The Intuitive SureForm Stapler 45 and 60, SureForm 45 and 60 reloads and other stapler accessories are intended to be used with a compatible da Vinci surgical system for resection, transection, and, or creation of anastomoses in General, Thoracic, Gynecologic, Urologic, and Pediatric surgery. The device can be used with staple line or tissue buttressing material (natural or synthetic).
Product names are trademarks or registered trademarks of Intuitive Surgical or their respective owners. See www.intuitive.com/trademarks.
Info Contact
| HEAD | OFFICE |
| Tel | +27 (11) 966 0600 |
| info@medhold.co.za | |
| Address | MSI Business Park, 68 Rigger Road, Spartan, Kempton Park, Gauteng, 1619 |